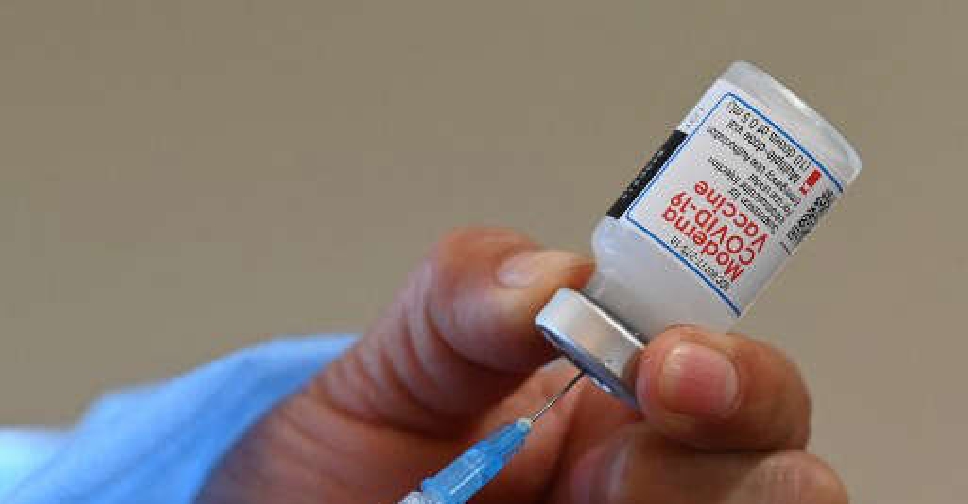
Britain's health regulator has approved Moderna's COVID-19 vaccine for use in children aged 12 to 17 years.
The approval comes more than two months after Pfizer and German partner BioNTech's COVID-19 vaccine got regulatory nod for use in children aged 12 to 15.
Moderna's vaccine was recommended for use in adolescents by European regulators in July and is awaiting US authorisation.
It is currently approved for people over the age of 18 in the UK.
Britain's Joint Committee on Vaccination and Immunisation (JCVI) gave the go-ahead on August 4 for 16 and 17-year-olds to get their first dose of Pfizer's COVID-19 vaccine ahead of schools returning in September.
JCVI will make a decision on whether the vaccine will be deployed or not.




 FBI foils "terror plot" targeting Los Angeles
FBI foils "terror plot" targeting Los Angeles
 Hong Kong court finds tycoon Jimmy Lai guilty in landmark security trial
Hong Kong court finds tycoon Jimmy Lai guilty in landmark security trial
 Ukraine peace talks stretch into second day at start of pivotal week for Europe
Ukraine peace talks stretch into second day at start of pivotal week for Europe
 Flash floods kill at least 37 people in Morocco's Safi province
Flash floods kill at least 37 people in Morocco's Safi province


